IAL EDEXCEL BIOLOGY (2018) UNIT 1 ( LAST MINUTE REVISION : Molecules, Diet, Transport and Health (Part 3)
Preface
- This series contains 5 parts which are divided into 2 videos for topic 1 - molecules, transport and health; 2 videos for topic 2 - membranes, proteins, DNA and genes expression; and biology practical.
- The revision will be based on the syllabus for (WBI11) IAL Edexcel Biology (2018) Unit 1
- The third part contains the first half of the syllabus for topic 2
Syllabus Checklist
1. Know the properties of gas exchange surfaces in living organisms (large surface area to volume ratio, thickness of surface and difference in concentration)
2. Understand how the rate of diffusion is dependent on these properties and can be calculated using Fick’s Law of Diffusion
3. Understand how the structure of the mammalian lung is adapted for rapid gaseous exchange
4. Know the structure and properties of cell membranes
5. Understand how models such as the fluid mosaic model of membrane structure are interpretations of data used to develop scientific explanations of the structure and properties of cell membranes
6. Understand what is meant by osmosis in terms of the movement of free water molecules through a partially permeable membrane, down a water potential gradient
7. Understand what is meant by passive transport (diffusion, facilitated diffusion), active transport (including the role of ATP as an immediate source of energy), endocytosis and exocytosis
8. Understand the involvement of carrier and channel proteins in membrane transport
9. Know the basic structure of an amino acid
10. Understand the formation of polypeptides and proteins (amino acid monomers linked by condensation reactions to form peptide bonds)
11. Understand the significance of a protein’s primary structure in determining its secondary structure, three-dimensional structure and properties (globular and fibrous proteins and the types of bonds involved in its three-dimensional structure)
12. Know the molecular structure of a globular protein and a fibrous protein and understand how their structures relate to their functions (including haemoglobin and collagen)
13. Understand the mechanism of action and the specificity of enzymes in terms of their three-dimensional structure
14. Understand that enzymes are biological catalysts that reduce activation energy
15. Know that there are intracellular enzymes catalysing reactions inside cells and extracellular enzymes catalysing reactions outside cells
1. Know the properties of gas exchange surfaces in living organisms (large surface area to volume ratio, thickness of surface and difference in concentration)
2. Understand how the rate of diffusion is dependent on these properties and can be calculated using Fick’s Law of Diffusion
3. Understand how the structure of the mammalian lung is adapted for rapid gaseous exchange
- Gas Exchange in Small Organisms
- Single-celled and very small multicellular organisms have a large surface area : volume ratio --> they can get oxygen they need for cellular respiration from the air or water they live in through their outer body surface.
- Gas Exchange in Large Organisms
- Large organisms are made up of millions of cells --> they have a small surface area : volume ratio --> diffusion alone may be too slow for nutrients or oxygen to diffuse from the outside to the inner cells --> hence they need a special mechanism to get nutrients faster
- The metabolism rate of large organisms tend to be higher than smaller organisms --> more demand on nutrient supply --> diffusion alone not quick enough --> need a special mechanism
- So in humans and many other large land animals, gas exchange takes place in the lungs
- In fish, it is the gills
- In insects, it is the tracheal system
- In plants, in the leaves
- Key properties of Gas Exchange Systems
- The rate of diffusion across a membrane in the gas exchange system is controlled by several factors:
- Surface area – the bigger the surface area, the more particles can be exchanged at the same time
- Concentration gradient of the particles diffusing – particles diffuse quicker from the concentration outside the cell is way higher than the concentration inside the cell (Steeper concentration gradient)
- Thickness of the gaseous exchange membrane – the shorter the diffusion distance, the quicker the diffusion
- Fick’s Law of Diffusion
- Features of an effective gaseous exchange system
- Large surface area for diffusion
- Large surface area : volume ratio
- Thin layers to minimize diffusion distance
- Rich blood supply to the respiratory surface
- Moist surface because diffusion takes place with the gases in solution
- Permeable surfaces to allow free movement
- Different parts of the breathing system
- What makes alveoli a great place for gaseous exchange?
- Breathing
- How to protect the lungs from pathogens or dust particles?
- The respiratory system produces lots of mucus that lines the airways and traps these tiny particles and organisms
- The mucus is very runny so it can be moved by the cilia within the airway into your throat to be swallowed
- The acid within the stomach kills the pathogens
4. Know the structure and properties of cell membranes
5. Understand how models such as the fluid mosaic model of membrane structure are interpretations of data used to develop scientific explanations of the structure and properties of cell membranes
- Structure of the cell membrane
- The Fluid Mosaic Model
- Definition – the current model of the structure of the cell membrane including floating proteins forming pores, channels and carrier system in a lipid bilayer
- Composed mainly of two types of molecules
- Phospholipids
- Proteins
- The phospholipid bilayer
- Phospholipids consists of a phosphate head and a fatty acid tail
- Why is it called the phospholipid bilayer?
- The phospholipid bilayer is made up of two layers of phospholipids
- The phosphate head faces the aqueous solution because of its hydrophilic nature and solubility in water
- The lipid tail faces the lipid tail of the other phospholipid molecule due to its nature which is hydrophobic and insoluble in water.
- This hence forms a double layered phospholipid.
- Properties of the phospholipid bilayer
- The layer allows the diffusion of fat-soluble organic molecules to pass through it because they are non-polar hence can pass through the lipid bilayer
- Polar molecules or molecules that can dissolve in water cannot pass through due to the lipid layer hence they require transport proteins
- Membrane Proteins
- Some proteins have a hydrophobic part, which is buried in the lipid bilayer, and a hydrophilic part which can be involved in a variety of activities
- Some proteins go all the way through the lipid bilayer
- Functions:
- Properties of cell membrane
- Act as a barrier which controls what passes through them --> allowing the fluids on either side to have different compositions --> makes it suitable to have the right reactions in the right place within the cell
- Contains enzymes and all factors needed for a reaction in close proximity to one another --> the reaction can go easily from one reaction to the next
- It is flexible --> allows the cell to change shape --> to accommodate the change in water content or for example in white blood cells, to engulf bacteria
- How scientists interpreted the results from their experiment?
- In addition
- The cell membrane also contains cholesterol.
- Function:
- These make the membrane more stable and stronger
- Makes it harder for small molecules and ions to pass through the membrane, so it acts as an effective barrier around the cell.
- Past paper practice questions
6. Understand what is meant by osmosis in terms of the movement of free water molecules through a partially permeable membrane, down a water potential gradient
7. Understand what is meant by passive transport (diffusion, facilitated diffusion), active transport (including the role of ATP as an immediate source of energy), endocytosis and exocytosis
8. Understand the involvement of carrier and channel proteins in membrane transport
- The main types of transport across membranes:
- Passive transport – takes place when there are concentration, pressure or electrochemical gradients, and involves no energy
- Active transport – involves moving substances into or out of the cell against their concentration gradient with the use of energy from ATP and involves a carrier protein.
- Passive Transport Mechanisms
- 3 main types
- Simple Diffusion
- Mechanism
- Molecules tend to move from an area of high concentration to an area of low concentration by the principle of random motion & kinetic energy of the molecules.
- For small molecules, like oxygen and carbon dioxide, the membrane is not a barrier so they can diffuse freely.
- However, hydrophilic molecules and ions which are larger than carbon dioxide molecules cannot move across the membrane by simple diffusion.
- Facilitated Diffusion
- Why?
- Some substances are polar (positive or negative charged) or large --> they cannot cross the cell membrane by simple diffusion --> therefore, they require a special way for diffusion known as facilitated diffusion
- Mechanism
- Facilitated diffusion involves proteins within the cell membrane
- The proteins may be channel proteins which act as pores
- Each type of channel protein allows one particular type of molecule to pass through
- The proteins may also be carrier proteins floating on the surface of the membrane
- Each type of carrier protein is specific to one particular type of molecules
- The molecule attaches to the active site of the protein carrier --> the carrier protein changes shape --> carries the molecule through the membrane --> and then releases the molecule into the opposite side of the membrane
- This process can only happen down the concentration gradient because it does not use energy
- Active Transport Mechanisms
- It requires energy to transport a molecule from an area of low concentration to an area of high concentration through a partially permeable membrane.
- Using a glucose molecule as an example
- Endocytosis and Exocytosis
- Why do we need endocytosis and exocytosis?
- Diffusion and active transport only allow small molecules to cross the membrane but not large particles.
- Mechanism:
- Endocytosis – membrane-bound vesicles surround and take up materials by phagocytosis (when engulfing large molecules) or pinocytosis (when engulfing liquid) and forms vesicles.
- Exocytosis – emptying of a membrane-bound vesicle at the surface of the cell --> releasing the molecules within the vesicles
- The formation and fusing of vesicles with the surface cell membrane are both active processes, requiring energy supplied by ATP.
9. Know the basic structure of an amino acid
10. Understand the formation of polypeptides and proteins (amino acid monomers linked by condensation reactions to form peptide bonds)
11. Understand the significance of a protein’s primary structure in determining its secondary structure, three-dimensional structure and properties (globular and fibrous proteins and the types of bonds involved in its three-dimensional structure)
12. Know the molecular structure of a globular protein and a fibrous protein and understand how their structures relate to their functions (including haemoglobin and collagen)
- Functions of Proteins:
- Make up the hair, skin and nails
- Make enzymes for metabolism and digestion
- Make hormones for endocrinal control
- Enables muscle fibres to contract
- Make antibodies
- Assist in blood clotting process
- Form haemoglobin in RBC for gaseous transport
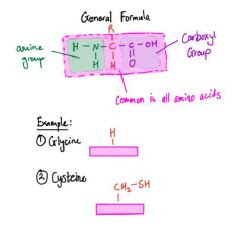
- Amino Acids
- Proteins are macromolecules made up of many monomers of amino acids
- There are about 20 different naturally occurring amino acids which can be combined together to produce different proteins
- All amino acids have the same basic structure
- An amino group (-NH2)
- A carboxyl group (-COOH)
- They also have an R group which may contain sulfur and selenium (R group determines the tertiary structure due to its polarity)
- Formation of proteins from amino acids
- They join by the reaction of amino group (-NH2) of one amino acid with the carboxyl group (-COOH) of another by condensation reaction and a water molecule as byproduct will be produced
- The bond between two amino acid is called the peptide bond
- 2 amino acids = dipeptide
- Many and many amino acids joined together = polypeptide
- This polypeptide folds due to bonding between R groups in amino acids which forms different shapes hence forming protein
- Bonds in Proteins
- Peptide bond is the strong bond between amino acids.
- Other three bonds depend on the R group of amino acids
- Peptide Bonds
- Hydrogen Bonds
- Tiny negative charges in oxygen of the carboxyl groups + tiny positive charges in hydrogen of the amino groups = Hydrogen bond
- They are weak
- They are very important for the folding and coiling of polypeptide chains.
- They are affected easily by a change in temperature and pH
- Disulfide Bonds
- Bond between two cysteine molecules
- An oxidation reaction occurs between the two sulfur-containing groups = disulfide bond
- They are important for holding the folded polpeptide chains in place
- Ionic Bonds
- Formed between some of the strongly positive amino acids and strongly negative amino acids
- Protein Structure
- Classified into primary, secondary, tertiary and quaternary structure:
- Primary structure
- The linear sequence of the amino acid chain held together only by peptide bonds
- Secondary structure
- Arrangement of the polypeptide chain into a regular, repeating three-dimensional (3D) structure, held together by hydrogen bonds between amino and carboxyl groups (not R group)
- Example: Fibrous proteins
- Tertiary structure
- Another level of 3D organization in addition to the secondary structure in many proteins.
- The secondary amino acid chain is folded into more complicated shapes
- The 3D structure is held together by hydrogen bonds, disulfide bonds and ionic bonds between R groups
- Example: Globular proteins
- Note:
- A small change in pH or temperature can cause the change in bonding between amino acids hence causing a change in 3D structure, this is called denaturation.
- The sequence of amino acids in the primary structure ultimately determines the bonding position and shape of the secondary and tertiary structures
- Fibrous and Globular Proteins
- Fibrous Proteins
- Characteristics
- Have little or no tertiary structure
- Are long, parallel polypeptide chains with occasional cross-linkages that form them into fibres
- Are insoluble in water and are very tough
- Example – Collagen
- Globular Proteins
- Characteristics
- Complex tertiary and sometimes quaternary structures
- Fold into spherical (globular) shapes
- Have a specific 3D shape which means they can be metabolically active as enzymes or hormones. If the shape is altered slightly by changing conditions, they lose their ability to function.
- Example: Haemoglobin, enzymes and hormones
- Conjugated Proteins
- Some protein molecules are joined with (conjugated to) another molecule called a prosthetic group.
- This structural feature usually affects the performance and functions of the molecules
- Examples of Conjugated Proteins:
- Haemoglobin = Iron + Protein
- Lipoprotein = Lipid + Protein
- Glycoprotein = Carbohydrates + Protein
13. Understand the mechanism of action and the specificity of enzymes in terms of their three-dimensional structure
14. Understand that enzymes are biological catalysts that reduce activation energy
15. Know that there are intracellular enzymes catalyzing reactions inside cells and extracellular enzymes catalyzing reactions outside cells
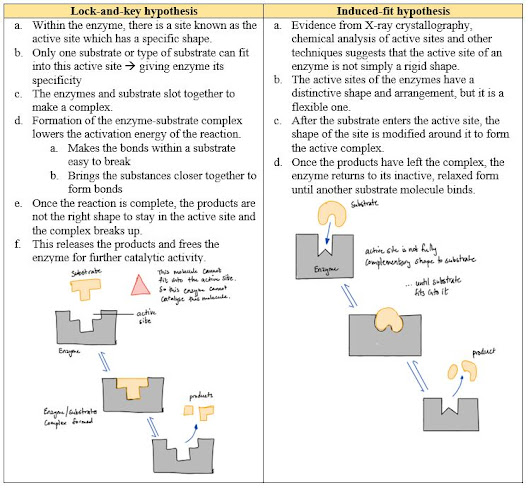
- How do enzymes work?
- Enzymes work by reducing the activation energy in a reaction
- The activation energy is the energy needed for a chemical reaction to get started.
- Characteristics of Enzymes:
- Globular proteins
- Contain an active site, which is essential for function
- The active site is a small depression that is of specific shape to the substrate
- Affected by pH and temperature --> changes to the 3D structure of the active site
- They act only to speed up the reaction
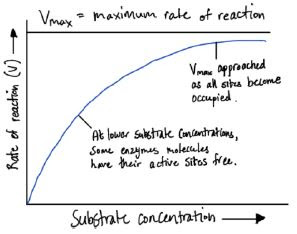
- Relationship between enzyme and substrate concentration:
- At the start, there are a lot more active sites than substrates
- As the number of substrates increase, the number of enzyme-substrate complex also increases.
- There comes a point where all active sites are occupied and further increasing the substrate concentration will not increase rate of reaction
- Relationship of temperature on the rate of enzyme activity:
- Rate of reaction increases with temperature at first because substrates and enzymes have the kinetic and potential energy to react.
- The optimum temperature is when the rate of reaction is at its peak (different enzymes have different optimum temperatures)
- Above the optimum temperature, the rate of reaction starts to decrease because the enzymes become denatured (loss of shape of active site) hence the substrates cannot bind to the site for reaction.
- Relationship of pH on the rate of enzyme activity:
- Different enzymes work in different ranges of pH
- This is because changes in pH affect the formation of the hydrogen bonds and disulfide bonds that hold the 3D structure of the active site.
- The graph is similar to the one for temperature.
- Definitions
Link to Youtube Video
Click here for Part 4





















Hi!! This is amazing and thank you for the work you do!! even if its just one person you've helped you're my superhero fr T-T!! is there any part 4 notes to this series?
ReplyDelete